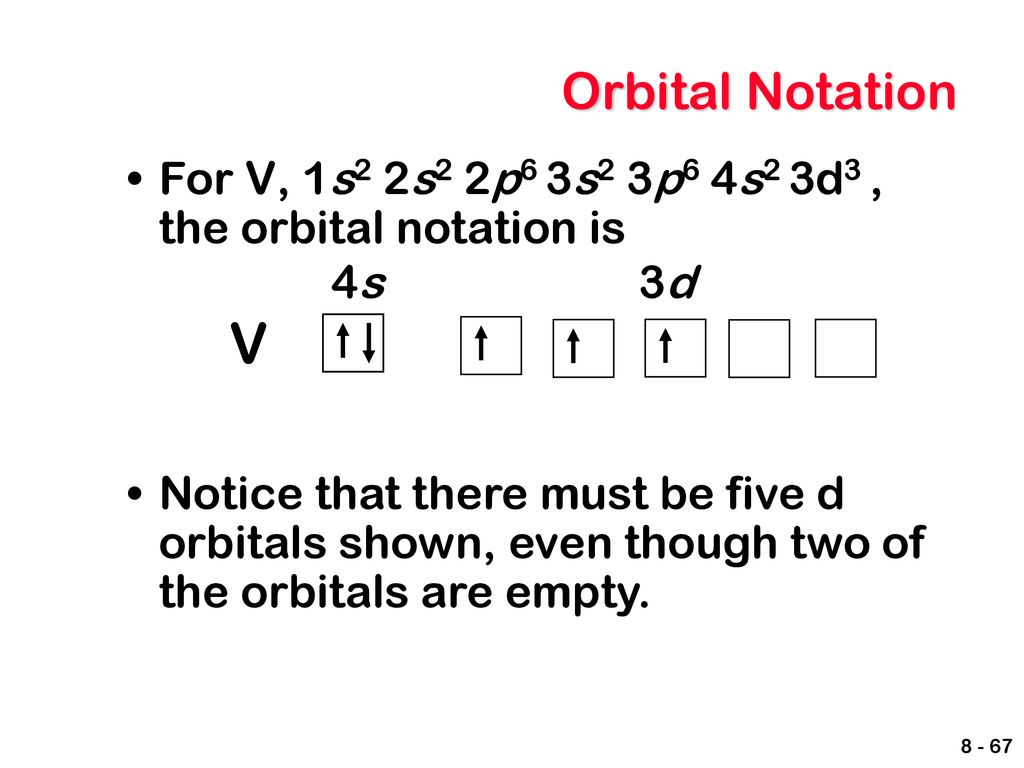
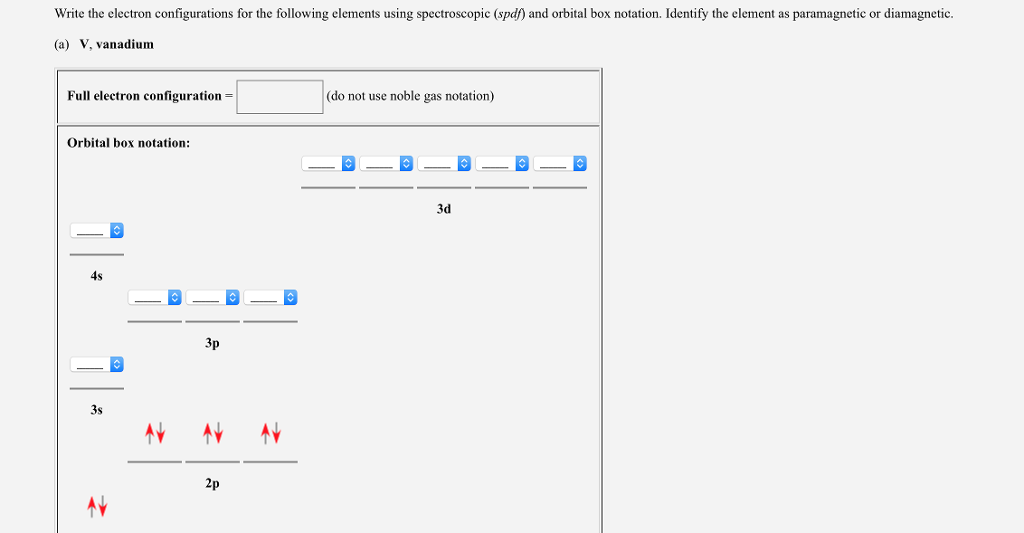
V Orbital Notation

Find out about its chemical and physical properties states energy electrons oxidation and more.
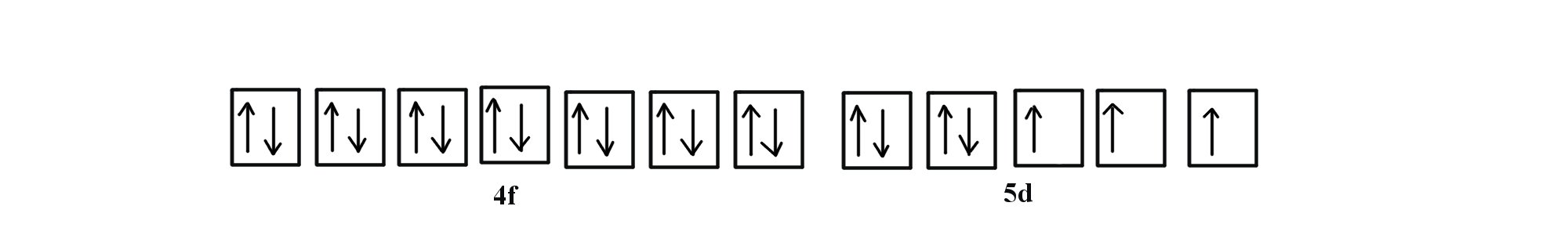
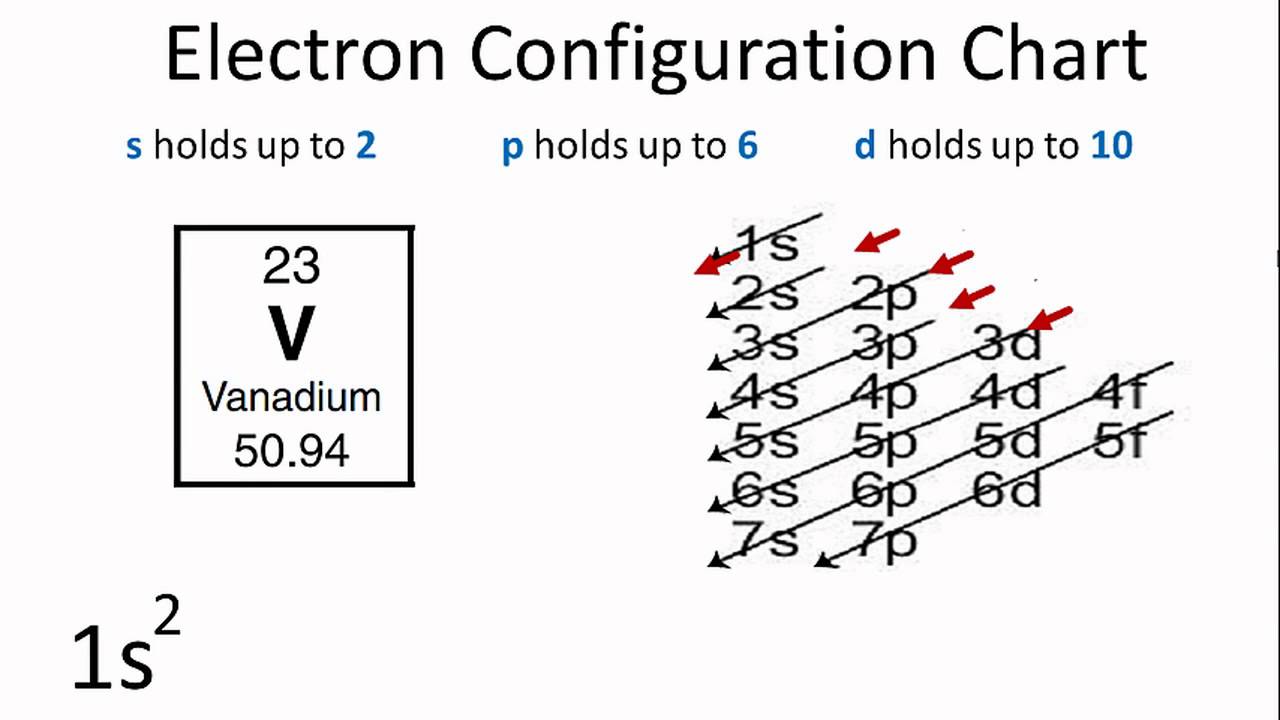
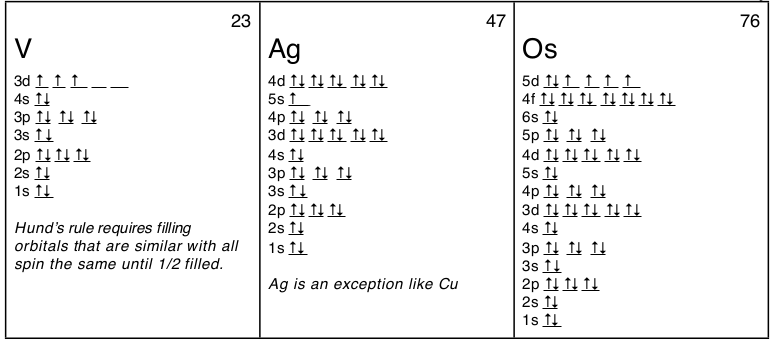
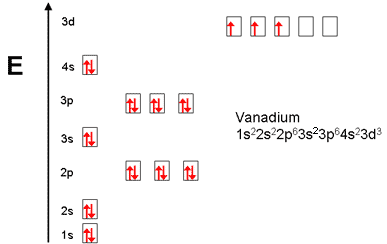
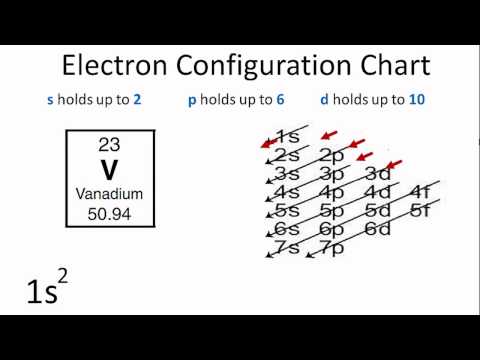
V orbital notation. Electron configuration for magnesium mg 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p2. This is a chemistry how to lesson on how to write the orbital notation using the electron configuration for an element. It turns out that the energy the electron configuration that is half filled 4s 1 3d 5 and filled orbital 4s 1 3d 10 has lower energy than the typical filling order 4s 2 3d 4 and 4s 2 3d 9 this pattern is followed in the 5 th row with mo 42 and ag 47. Electron configuration for vanadium v 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d1.
Vanadium v has an atomic mass of 23. Also displays electrons as half arrows oriented up and down. Does not show the relative energies of orbitals for a single atom but does at least show their ordering. Periodic table exceptions to know.
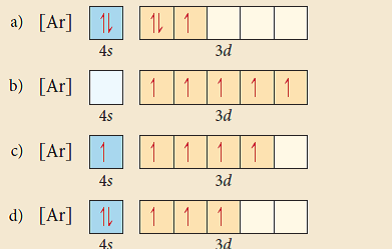
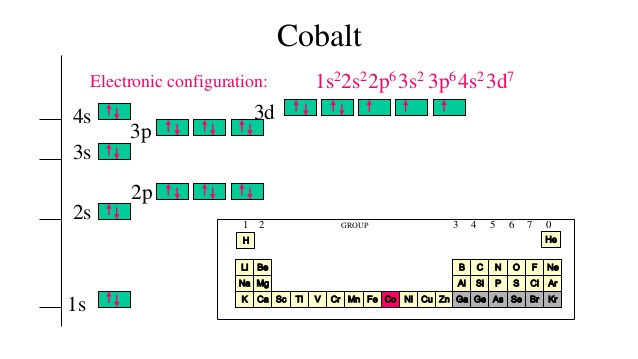
It is very useful in determining quantum numbers same as electron pairing. This is sometimes called the orbital. Orbital notation orbital notation is a drawing of the electron configuration. There is a major exception to the normal order of electron configuration at cr 24 and cu 29.
1s2 2s2 2p6 3s2. Orbital elements are the parameters required to uniquely identify a specific orbit in celestial mechanics these elements are considered in two body systems using a kepler orbit there are many different ways to mathematically describe the same orbit but certain schemes each consisting of a set of six parameters are commonly used in astronomy and orbital mechanics. Electron configuration for yttrium y carbon c. Orbital notation is just another way of expressing the electron configuration of an atom in a visual way.
But first you must apply hund s rule of maximum multiplicity.