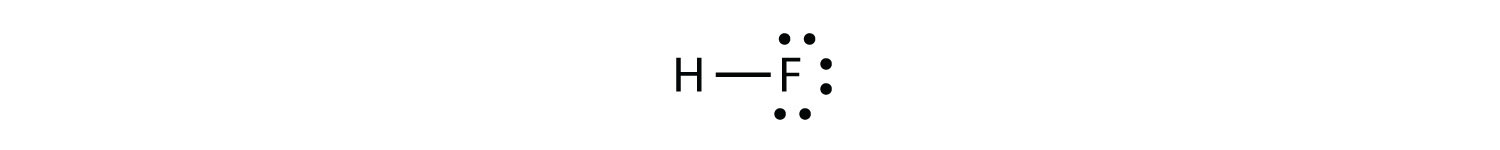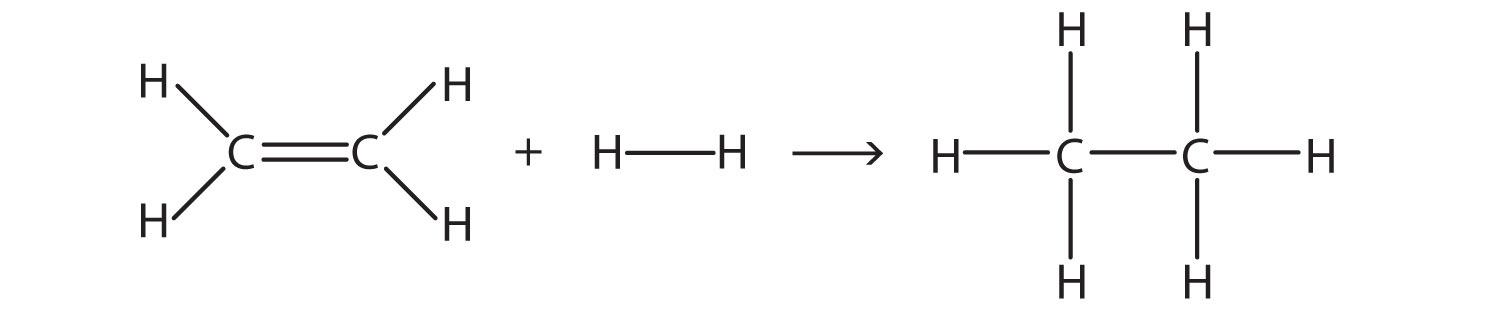Rb And N Bond Type

Non polar covalent 0.
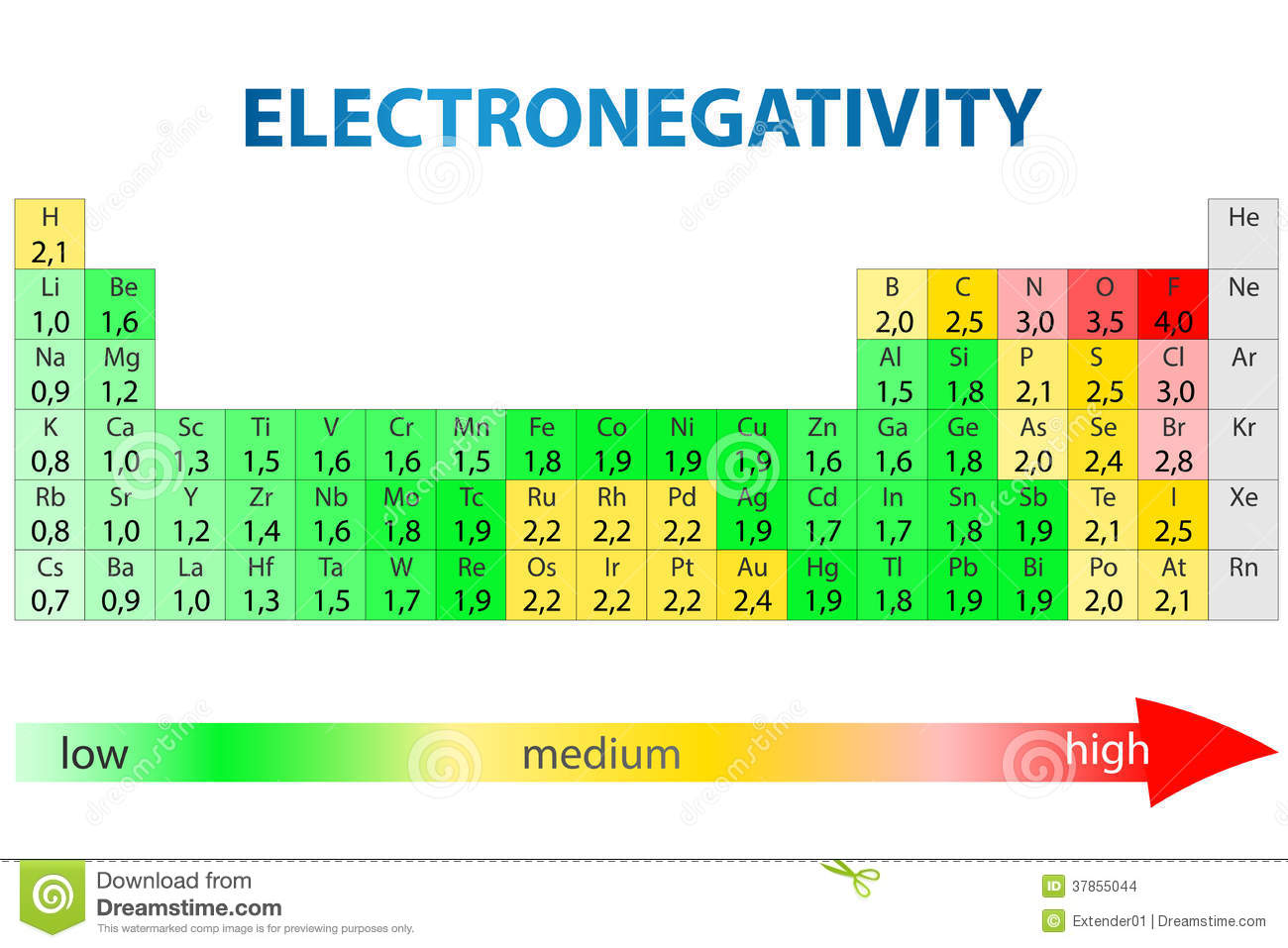
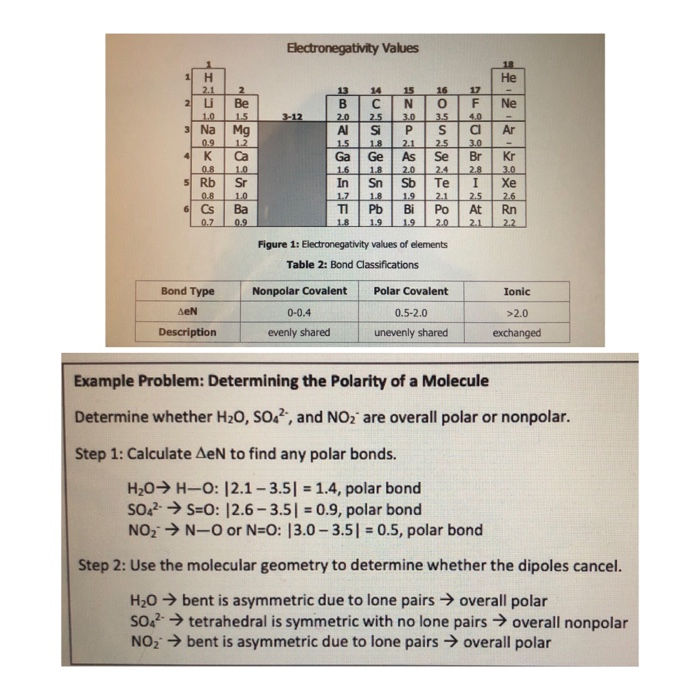
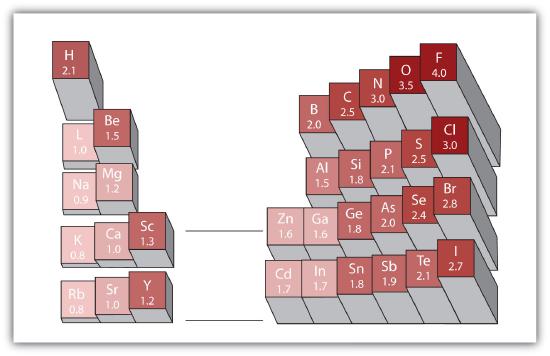
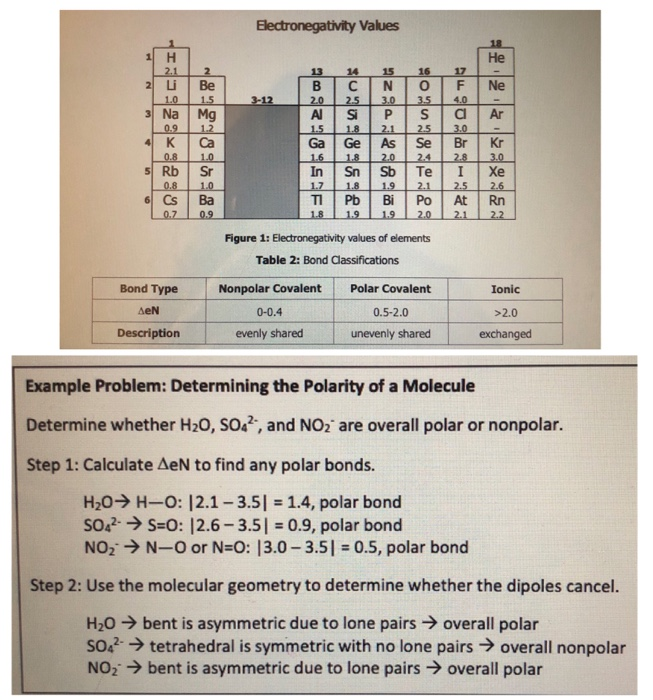
Rb and n bond type. Non polar covalent 0. Covalent bonding is a type of chemical bonding where electrons of two or more atoms share common electrons between them. What kind of bond. How is the n br bond described.
Calculate the bond type and molecular polarity of rubidium monochloride clrb based on the electronegativity of the atoms. There are two main types of bonds. The si f is one of the most polarized covalent bonds and has a lot of ionic character. These are the safest bonds of all because the interest and principal payments are guaranteed by the u s.
There is actually a continuum of bond types. Calculate the bond type and molecular polarity of phosphorus mononitride pn based on the electronegativity of the atoms. Rb cool bond m appaloosa 2011 rb cool bond 2011 appaloosa. Coolest dream sor 15 1 hh n n 2002.
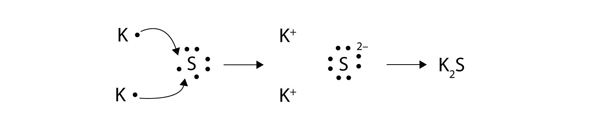
For example a c c covalent bond has little or no ionic character because the two atoms have the same en s. Types of bonds march 24 2017. Ionic non covalent first element. Covalent and ionic bonds.
The n li bond is ionic but has significant. A covalent bond can be polar or nonpolar but not ionic. Most bonds have some covalent and some ionic character. 0 polar covalent 2.