L Amino Acid Oxidase

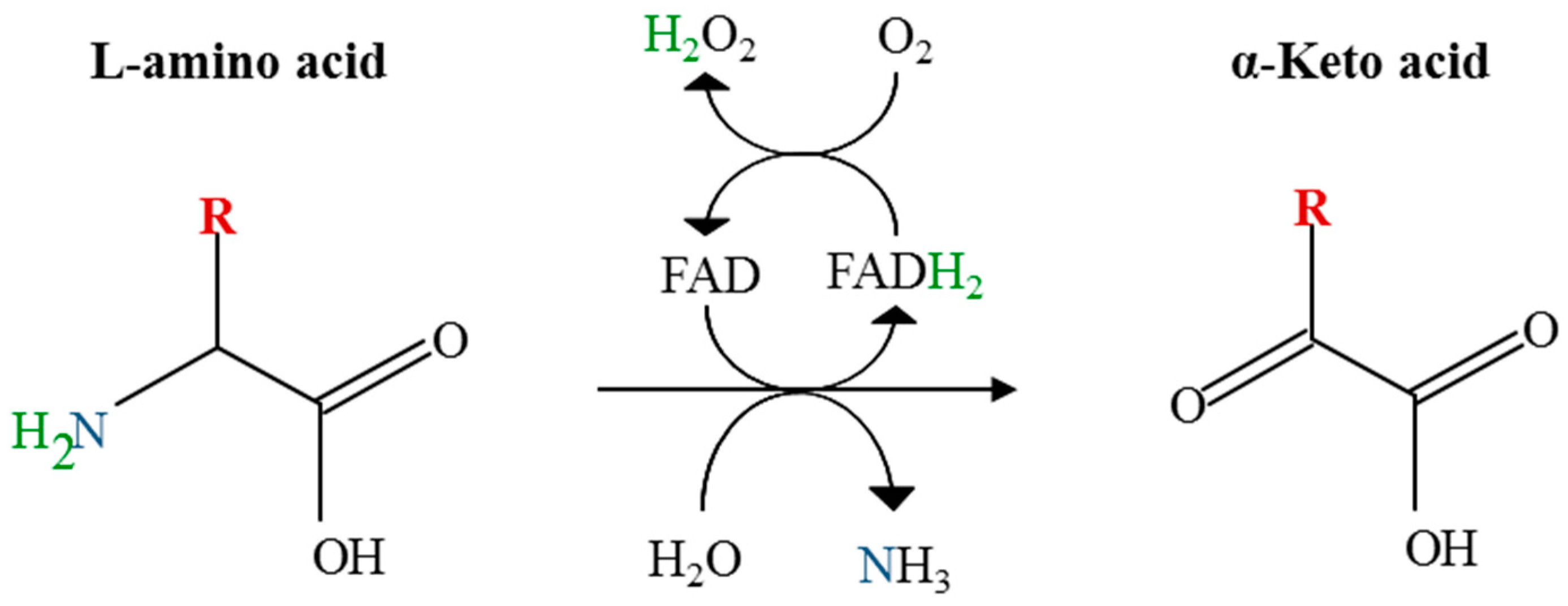
In enzymology an l amino acid oxidase laao ec 1 4 3 2 is an enzyme that catalyzes the chemical reaction.
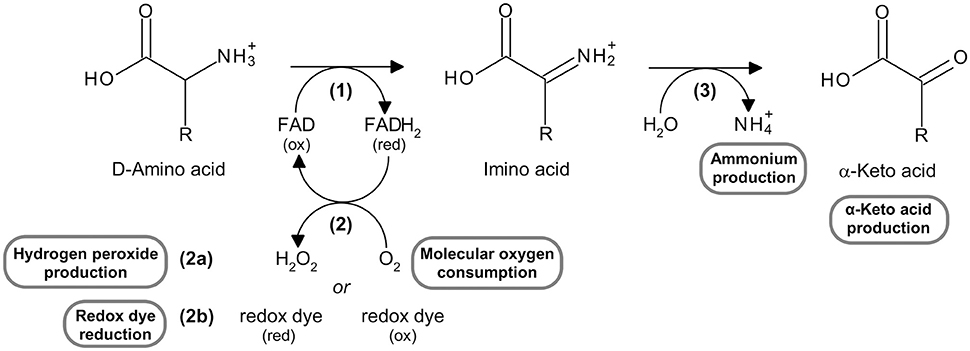
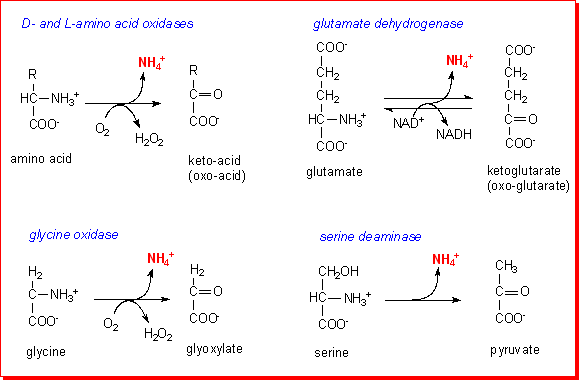
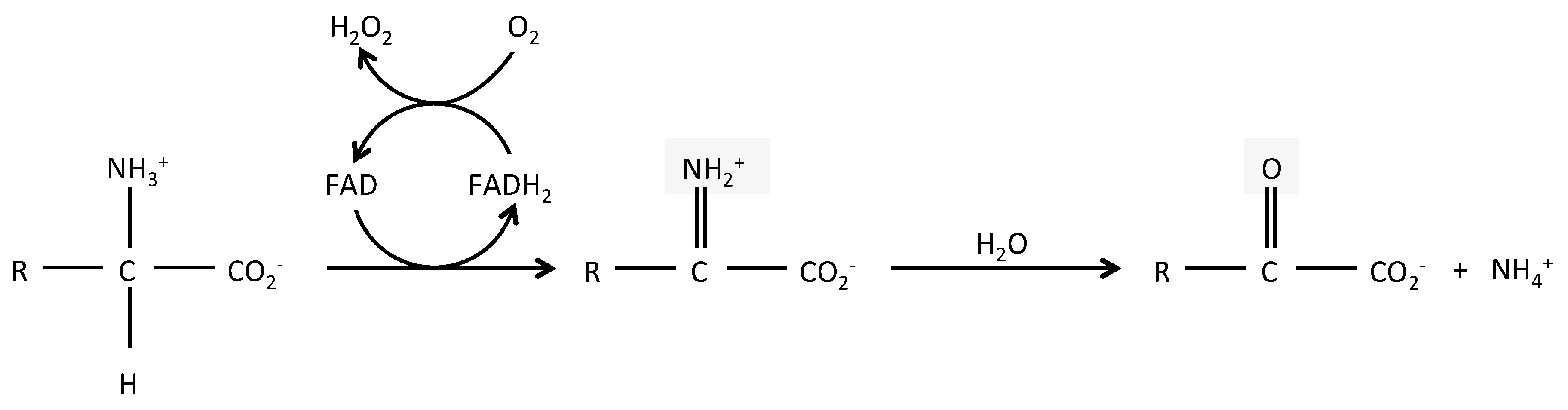
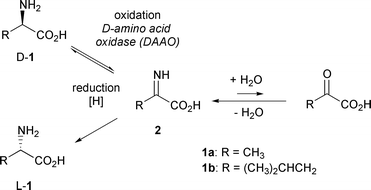
L amino acid oxidase. This score cannot be used as a measure of the accuracy of the annotation as we cannot define the correct annotation for any given protein. Also oxda damox is an enzyme with the function on a molecular level to oxidize d amino acids to the corresponding α keto acids producing ammonia and hydrogen peroxide this results in a number of physiological effects in various systems most notably the brain. Reagent a buffer 11 70 reagent b l phe 3 00 reagent d boric acid 14 00 mix by stirring and adjust to ph 6 5 at 37 c with 1 m hcl or 1 m naoh if necessary. The digestion of dietary protein and catabolism by enzymes in the enterocytes and gut microflora produce ammonium ions.
Prepare a reaction cocktail by pipetting in milliliters the following reagents into a suitable container. There are approximately two moles of fad per mole of holo enzyme. The enzyme is most active toward neutral d amino acids and not active toward acidic d amino acids. Its substrates include a wide variety of d amino acids but it is inactive on the naturally occurring l amino acids.
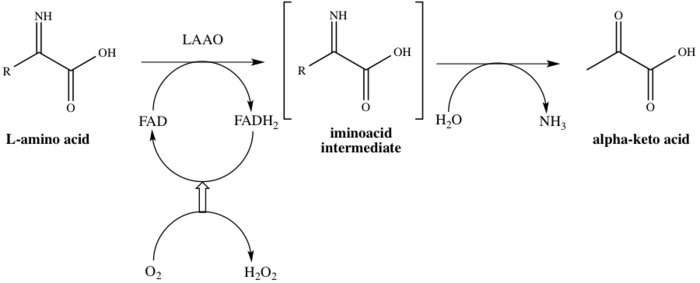
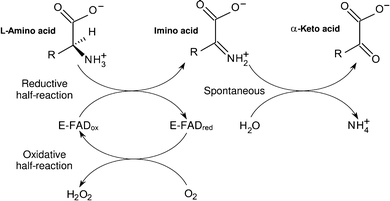
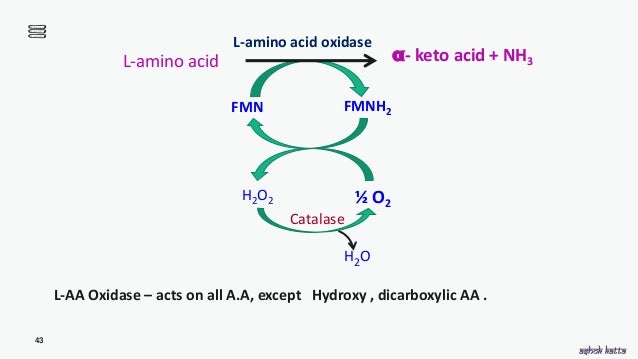
Laao was first discovered by zeller and maritz zeller and maritz 1944 1945. Laao represents approximately 30 of the total venom of some snake species takatsuka et al. The enzyme was first described in 1944 by a. L amino acid oxidase laao is a flavoenzyme containing non covalently bound flavin adenine dinucleotide which catalyzes the stereospecific oxidative deamination of l amino acids to α keto acids and also produces ammonia and hydrogen peroxide via an imino acid intermediate.
An l amino acid h 2 o o 2 a 2 oxo acid nh 3 h 2 o 2. This gene encodes the peroxisomal enzyme d amino acid oxidase. Three electrophoretically different isozymes occur as different combinations of the two subunits. A heat stable form of l amino acid oxidase isolated from king cobra ophiophagus hannah venom oh laao has been shown to exhibit very potent cytotoxicity against human tumorigenic cells but not in their non tumorigenic counterparts and the cytotoxicity was due to the apoptosis inducing effect of the enzyme.
D amino acid oxidase daao. L amino acid oxidase and d amino acid oxidase are minor sources of ammonium ions in the body. L amino acid oxidase laao catalyzes the oxidative deamination of a number of l amino acids predominantly hydrophobic and aromatic l amino acids. The enzyme is a flavoprotein which uses flavin adenine dinucleotide fad as its prosthetic group.
Apparently most blood ammonia originates directly from the catabolism of dietary protein in the gut.