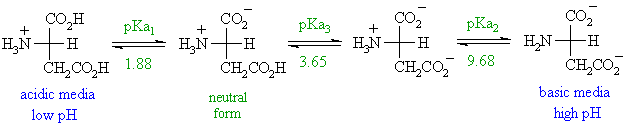L Amino Acid In Zwitterion Form
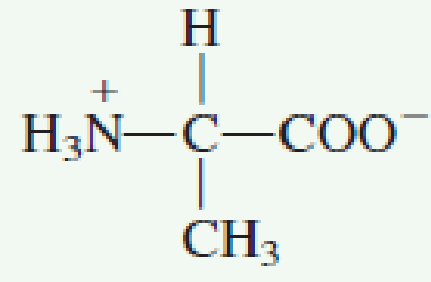
Dipole bound excess electrons attached to amino acids or amino acid solvent clusters stabilize the zwitterionic form of amino acids relative to their canonical forms.
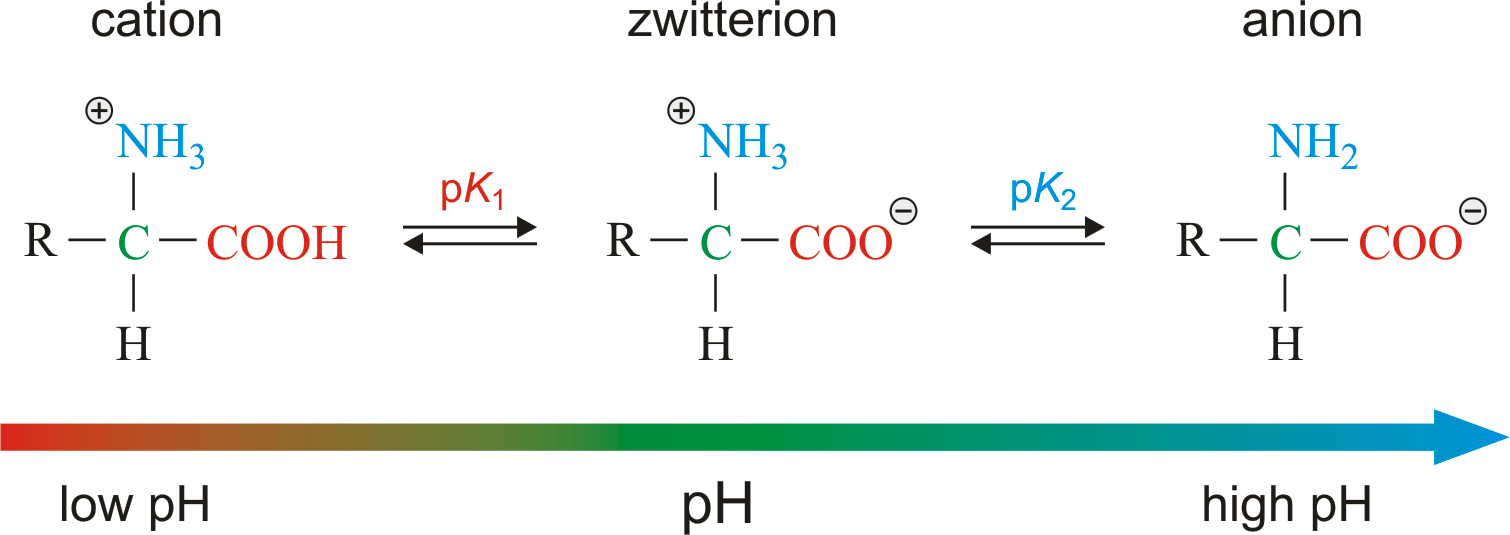
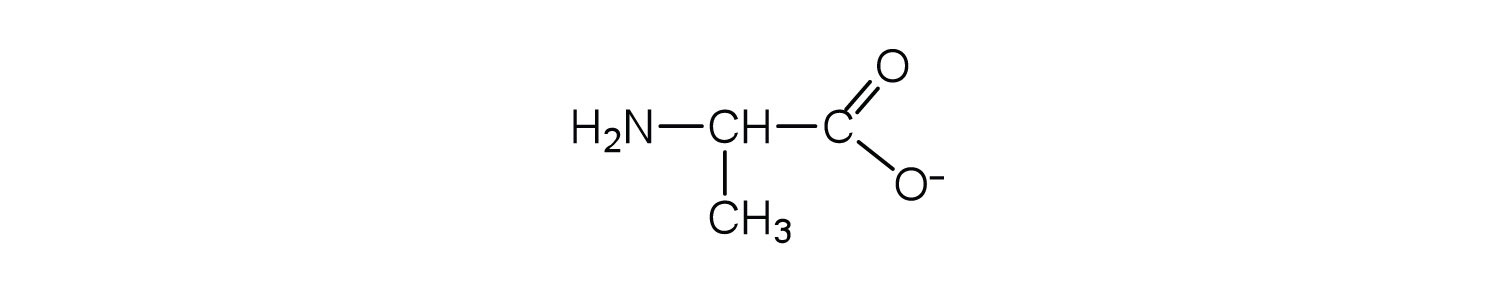
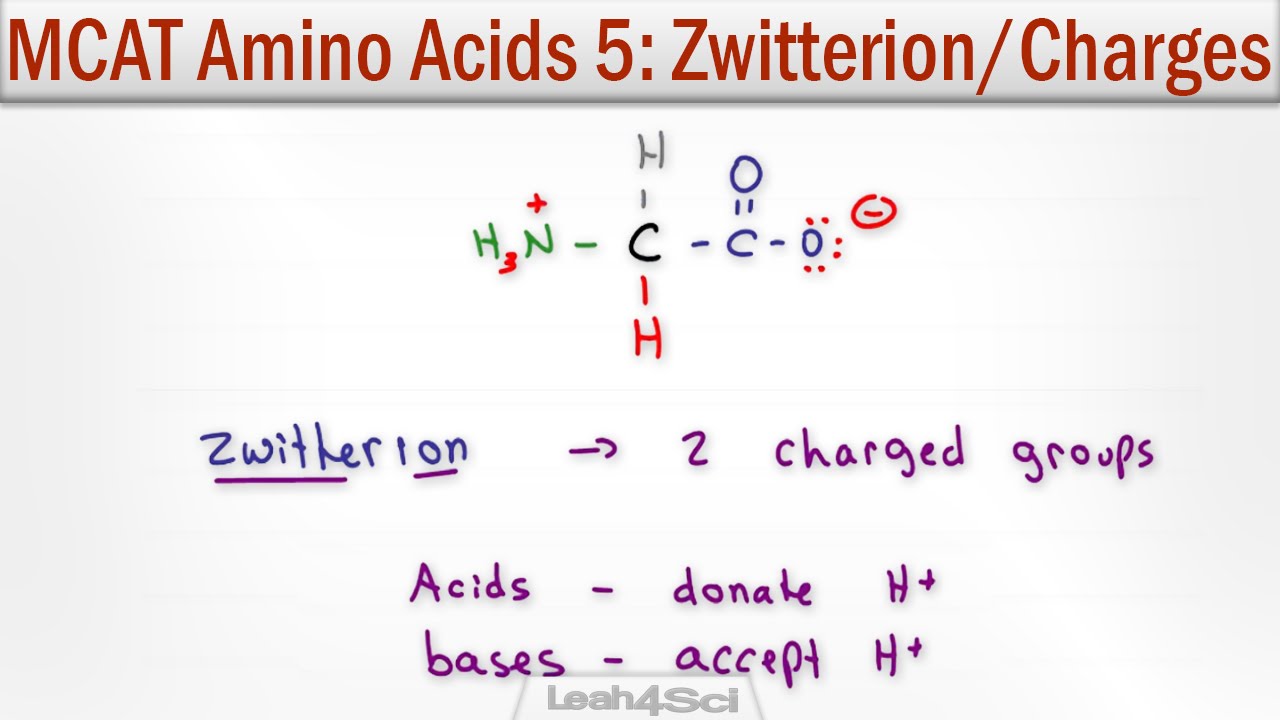
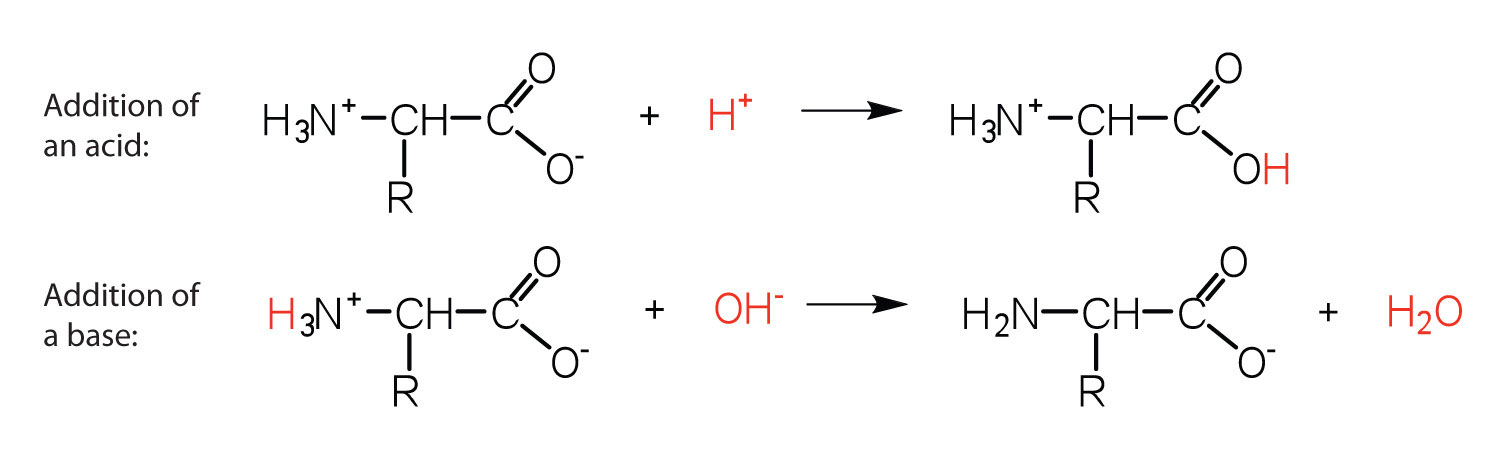
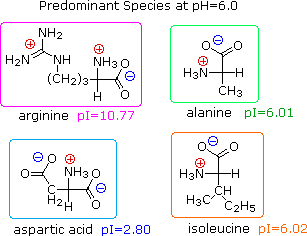
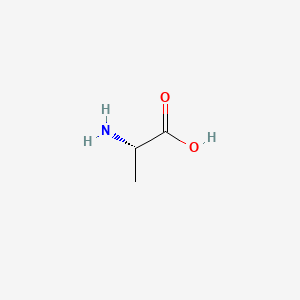
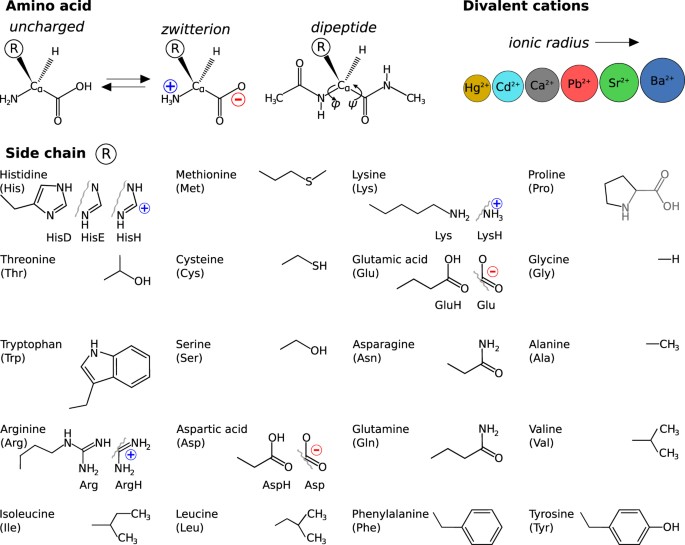
L amino acid in zwitterion form. While neutral the zwitterion form of an amino acid will have a positive and a negative charge. Every zwitterion has an isoelectric point pi. Amphoteric molcules are not necessarily zwitterionic. Alanine has a non protic side chain a methyl and thus at ph 7 4 physiological ph the carboxyl group has a negative charge coo and the amino group has a positive charge rnh3.
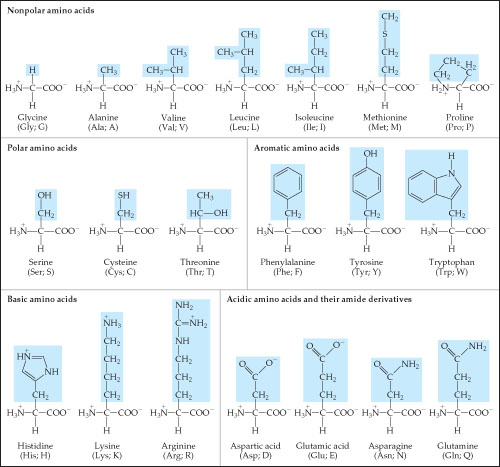
With two dissociation steps controlled by two acidity constants k 1 and k 2. This has been predicted 357 and experimentally verified 344 in the case of arginine. Zwitterion comes from the german word for two zwei or double zwitter and the word ion. The r group represents the side chain of different amino acids.
Arginine plus an excess electron indeed exist as a zwitterion in low temperature gas phase. Nc o c c h nh3 c o o. L asparagine zwitterion chebi 58048 is a amino acid zwitterion chebi 35238 l asparagine zwitterion chebi 58048 is tautomer of l asparagine chebi 17196 incoming. Note the diprotic amino acid alanine.
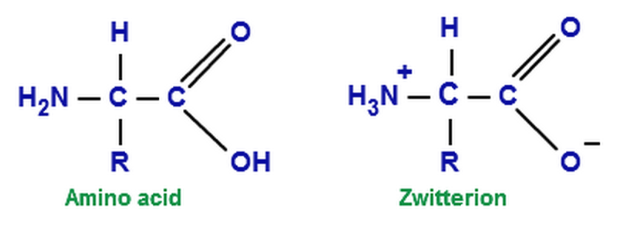
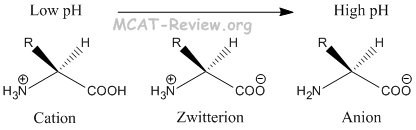
The standard structure contains both a carboxyl and an amine in the backbone. This is the zwitterion form of an amino acid. The neutral zwitterion is the usual form amino acids exist in solution. The amino group of an.
The ph can affect the charge of a molecule by introducing protons h. The isoelectric point is the ph at which a zwitterion is uncharged. The zwitterion form of an amino acid is shown below.